Multiple Sclerosis Center
Since 1993, our MS Center has worked to provide state-of-the-art neurological consultation and care for persons with multiple sclerosis in North Texas and the surrounding area. In addition to clinical activities, the MS Center is dedicated to all phases of clinical research for new, potential MS treatments from first-in-man Phase I through Final Phase III studies for FDA review.
The Care Team
What is Multiple Sclerosis?
Multiple Sclerosis (MS) is a chronic, inflammatory and neurodegenerative human disease which results from the autoimmune destruction of myelin and associated collateral tissue damage within the human central nervous system.
MS affects approximately 2.5 million persons worldwide. In the United States, MS is the most common cause of non-traumatic, long-term neurological disability in young adults, affecting at least 400,000 persons. Although MS is found throughout the world, marked regional variability is well known and likely reflects a combination of multiple interacting genetic and environmental influences. MS is usually first noted in young adults, affecting women two to three times more frequently than men.
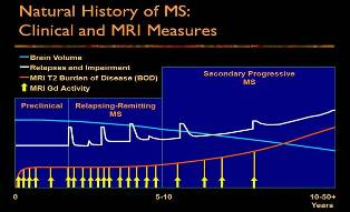
Figure. The Nature History of MS: Clinical and MRI Features (Compiled from numerous sources.)
Despite the common description of MS as one disease, several subtypes are recognized clinically. The established clinical subtypes include: relapsing-remitting (RRMS), primary progressive (PPMS), secondary progressive (SPMS), and progressive-relapsing (PRMS) forms. Over decades most untreated persons with RRMS will become ?secondarily progressive?; i.e. will develop SPMS. At least 85% of the entire MS population is represented within the combined categories of RRMS and its subsequent form, SPMS. Persons with primary progressive MS (PPMS) are those who never have relapses and remissions, and comprise about 10% of all persons with MS. Progressive-relapsing MS (PRMS) is progressive from the onset without remission and is later punctuated with relapses and remissions. This form of MS affects about 5% of persons with MS.
A common individual course of MS is shown in the figure. As shown, the first clinical evidence of RRMS is usually preceded by subclinical disease activity most sensitively detected by changes in magnetic resonance imaging (MRI) of the brain. Eighty to 90% of new MRI events may not cause clinical symptoms perhaps due to their relatively small size, preferential distribution within the brain white matter, and redundancy of brain circuitry.
A new MRI event is an area of inflammation which is typically detected with a gadolinium (Gd)-enhanced MRI of the brain or spinal cord. After a period of a few weeks, the inflammation subsides, leaving an area of scar tissue (sclerosis) behind. The descriptive diagnostic term ?multiple sclerosis? derives from the multiplicity of these scarred (sclerotic) areas accumulated over time.
Clinical relapses can be associated with a variety of symptoms due to MS-related damage in brain and spinal cord. These may include disturbances of sensation, limb weakness, changes in vision or speech, balance or coordination problems, bowel and bladder control problems, excessive fatigue, and cognitive problems. As more inflammatory events occur over time, some of which actually reach clinical prominence (clinical relapses), the overall clinical and MRI extent of disease mounts (as shown in the Figure, white and orange lines, respectively).
At the same time, and now known to begin at even the earliest stages of MS, brain tissue loss also starts to occur, especially in untreated individuals. These changes correlate with long term irreversible physical and cognitive disabilities seen in many persons with MS. After 10 years disease duration, approximately 50% of untreated RRMS individuals will transition to SPMS.
This evolution is currently only recognizable after slow clinical worsening is noted in the absence of clinical relapses. All of these processes together lead to gradual MS worsening such that half of untreated persons with MS require a walking aid after 15-20 years of illness. The goals of early treatment of persons with MS are to help prevent the accumulation of brain and spinal cord damage that results in the disabling features of MS.
What causes MS?
The answer still remains obscure. Many studies suggest that both genetic and environmental influences play important interacting roles in determining risk for developing MS. MS prevalence varies with geographic location such that MS is more common in high northern or southern latitude regions and less common in equatorial regions. This peculiar finding is perhaps due in part to emigration patterns of higher risk groups, but most recently have been suggested to be related to less sun exposure and abnormalities in vitamin D metabolism more prominent at higher latitudes.
Various infectious causes have also been considered over the last 60 years, although none have shown a clear association with the disease. Recently, attention has been re-focused on the Epstein-Barr virus as a possibly important influence early in life, but there is no evidence that MS is infectious. Neither is MS is an inherited illness in any classic sense, but genes do appear to play some role in determining susceptibility to acquiring MS.
Disease Modifying Agents (DMAs)
In 1993, the first disease-modifying treatment for MS was approved by the US FDA (interferon beta-1b subcutaneous; Betaseron). Since then, five additional disease modifying agents have been approved for use in MS:
- Avonex (interferon beta-1a intramuscular)
- Copaxone (glatiramer acetate; copolymer-1; Cop-1)
- Rebif (interferon beta-1a subcutaneous)
- Novantrone (mitoxantrone intravenous)
- Tysabri (natalizumab intravenous)
These agents are called "disease modifying" because each is capable of altering the individual's MS course for the better. Specifically, the approved DMAs all tend to suppress further worsening of MS, particularly regarding relapses.
As of 2008, approximately 20 new possible DMAs are in Final Phase III testing. These include new agents attacking many different aspects of MS, and these also include oral medications. The future holds true promise for developing new, effective, safe, and more convenient treatments for MS. New and exciting approaches to repair MS-related brain and spinal cord damage are on the near horizon. The future, we feel, is bright and encouraging.
Clinical Trials
-
Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Two Year Study to Evaluate the Effect of Subcutaneous RO4909832 on Cognition and Function in Prodromal Alzheimer's Disease With Option for up to an Additional Two Years of Treatment and an Open-Label Extension With Active Study Treatment
Recruitment StatusClosedConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: Hoffmann-La RocheMore InformationMore Information: Click here to learn more. -
A Placebo-Controlled, Double-Blind, Parallel-Group, Bayesian Adaptive Randomization Design and Dose Regimen-finding Study With an Open-Label Extension Phase to Evaluate Safety, Tolerability and Efficacy of BAN2401 in Subjects With Early Alzheimer's Disease
Recruitment StatusActive: Not RecruitingConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: Eisai Inc.More InformationMore Information: Click here to learn more. -
Assessment of Safety, Tolerability and Efficacy of LY3002813 in Early Symptomatic Alzheimer's Disease
Recruitment StatusClosedConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: Eli Lilly and CompanyMore InformationMore Information: Click here to learn more. -
A Placebo-Controlled, Double-Blind, Parallel-Group, 18-Month Study With an Open-Label Extension Phase to Confirm Safety and Efficacy of BAN2401 in Subjects With Early Alzheimer's Disease
Recruitment StatusActive: Not EnrollingConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: Eisai Inc.More InformationMore Information: Click here to learn more. -
Randomized, Double-Blind, Placebo-Controlled, Three-Arm, 12-Month, Safety and Efficacy Study of TRx0237 Monotherapy in Subjects With Alzheimer's Disease Followed by a 12-Month Open-Label Treatment
Recruitment StatusClosedConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: TauRx Therapeutics LtdMore InformationMore Information: Click here to learn more. -
A Phase 3, Multicenter, Long Term, Extension Study of the Safety and Efficacy of AVP-786 (Deuterated [d6] Dextromethorphan Hydrobromide [d6-DM]/Quinidine Sulfate [Q]) for the Treatment of Agitation in Patients With Dementia of the Alzheimer's Type
Recruitment StatusClosedConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: Avanir PharmaceuticalsMore InformationMore Information: Click here to learn more. -
A Phase III, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy, and Safety Study of Gantenerumab in Patients With Early (Prodromal to Mild) Alzheimer's Disease
Recruitment StatusActive: Enrollment ClosedConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: Hoffmann-La RocheMore InformationMore Information: Click here to learn more. -
HEALEY ALS Platform Trial
Recruitment StatusActive: RecruitingConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: NEALSMore InformationMore Information: Click here to learn more. -
A Phase 3, Multi-Center, Double-Blind, Randomized, Placebo-Controlled Trial to Evaluate the Efficacy and Safety of Reldesemtiv in Patients with Amyotrophic Lateral Sclerosis (ALS)
Recruitment StatusActive: RecruitingConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: CytokineticsMore InformationMore Information: Click here to learn more. -
PLS Natural History (PNHS) Study
Recruitment StatusActive: RecruitingConditionCondition:SponsorCondition: Amyotrophic Lateral Sclerosis (ALS)More InformationMore Information: Click here to learn more. -
TRx0237 for the Treatment of Early and Mild-Moderate Alzheimer's Disease: Individual Patient Use
Recruitment StatusClosedConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: TauRx Therapeutics LtdMore InformationMore Information: Click here to learn more. -
A Multicenter, Open Label, Multiple Dose, Parallel Group Investigation Of The Long-term Safety, Tolerability, Reactogenicity, Pharmacokinetics And Pharmacodynamics Of Aab-001 Administered Subcutaneously In Subjects With Mild To Moderate Alzheimer's Disease
Recruitment StatusCompleted: 2012ConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: PfizerMore InformationMore Information: Click here to learn more. -
A Phase 3 Extension, Multicenter, Long Term Safety And Tolerability Trial Of Bapineuzumab (Aab 001, Eln115727) In Subjects With Alzheimer Disease Who Are Apolipoprotein E 4 Carriers And Participated In Study 3133k1-3001-us Or Study 3133k1-3001-ww.
Recruitment StatusCompleted: 2012ConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: PfizerMore InformationMore Information: Click here to learn more. -
A Phase 3 Extension, Multicenter, Double-Blind, Long-Term Safety and Tolerability Treatment Trial of Bapineuzumab (AAB-001, ELN115727) in Subjects With Alzheimer's Disease Who Participated in Study ELN115727-301 or Study ELN115727-302.
Recruitment StatusCompleted: 2012ConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: JANSSEN Alzheimer Immunotherapy Research & Development, LLCMore InformationMore Information: Click here to learn more. -
A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy and Safety Trial of Bapineuzumab (AAB-001, ELN115727) In Patients With Mild to Moderate Alzheimer's Disease Who Are Apolipoprotein E4 Carriers.
Recruitment StatusCompleted: 2012ConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: JANSSEN Alzheimer Immunotherapy Research & Development, LLCMore InformationMore Information: Click here to learn more. -
A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy and Safety Trial of Bapineuzumab (AAB-001, ELN115727) In Patients With Mild to Moderate Alzheimer's Disease Who Are Apolipoprotein E4 Non- Carriers.
Recruitment StatusCompleted: 2012ConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: JANSSEN Alzheimer Immunotherapy Research & Development, LLCMore InformationMore Information: Click here to learn more. -
A Phase Iii, Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel Group, Efficacy And Safety Trial Of Bapineuzumab (Aab 001, Eln115727) In Subjects With Mild To Moderate Alzheimer Disease Who Are Apolipoprotein E 4 Carriers
Recruitment StatusCompleted: 2012ConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: PfizerMore InformationMore Information: Click here to learn more. -
A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel-group Efficacy And Safety Trial Of Bapineuzumab (Aab-001, Eln115727) In Subjects With Mild to Moderate Alzheimer Disease Who Are Apolipoprotein E 4 Non-carriers
Recruitment StatusCompleted: 2013ConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: PfizerMore InformationMore Information: Click here to learn more. -
A Phase 3 Randomized, Double-blind, Placebo-Controlled Study of the Safety and Effectiveness of Immune Globulin Intravenous (Human), 10% Solution (IGIV, 10%) for the Treatment of Mild to Moderate Alzheimer's Disease
Recruitment StatusCompleted: 2013ConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: Baxalta now part of ShireMore InformationMore Information: Click here to learn more. -
A Seamless Phase IIa/IIb, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel Group Trial to Evaluate the Efficacy and Safety of MK-7622 as an Adjunctive Therapy for Symptomatic Treatment in Subjects With Alzheimer's Disease
Recruitment StatusCompleted: 2016ConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: Merck Sharp & Dohme Corp.More InformationMore Information: Click here to learn more. -
A Randomized, Placebo Controlled, Parallel-Group, Double Blind Efficacy and Safety Trial of MK-8931 With a Long Term Double-Blind Extension in Subjects With Mild to Moderate Alzheimer's Disease (Protocol No. MK-8931-017-10)(Also Known as SCH 900931, P07738)
Recruitment StatusCompleted: 2017ConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: Merck Sharp & Dohme Corp.More InformationMore Information: Click here to learn more. -
Effect of Passive Immunization on the Progression of Mild Alzheimer's Disease: Solanezumab (LY2062430) Versus Placebo
Recruitment StatusCompleted: 2017ConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: Eli Lilly and CompanyMore InformationMore Information: Click here to learn more. -
A 26-Week, Double-blind, Randomized, Placebo-controlled, Parallel-group Study to Investigate the Effects of Daily Administration of AC-1204 in Participants With Mild to Moderate Alzheimer's Disease (AD) With an Optional 26-Week Open Label Extension
Recruitment StatusCompleted: 2017ConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: CerecinMore InformationMore Information: Click here to learn more. -
A 24-month, Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel-group, Efficacy, Safety, Tolerability, Biomarker, and Pharmacokinetic Study of AZD3293 in Early Alzheimer's Disease (The AMARANTH Study)
Recruitment StatusCompleted: 2018ConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: AstraZenecaMore InformationMore Information: Click here to learn more. -
A Phase III Randomized, Placebo-Controlled Clinical Trial to Study the Safety and Efficacy of Suvorexant (MK-4305) for the Treatment of Insomnia in Subjects With Alzheimer's Disease
Recruitment StatusCompleted: 2018ConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: Merck Sharp & Dohme Corp.More InformationMore Information: Click here to learn more. -
A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled Study to Assess the Efficacy, Safety, and Tolerability of AVP-786 (Deuterated [d6]-Dextromethorphan Hydrobromide [d6-DM]/Quinidine Sulfate [Q]) for the Treatment of Agitation in Patients With Dementia of the Alzheimer's Type.
Recruitment StatusCompleted: 2019ConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: Avanir PharmaceuticalsMore InformationMore Information: Click here to learn more. -
A Phase III, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy and Safety Study of Crenezumab in Patients With Prodromal to Mild Alzheimer's Disease
Recruitment StatusCompleted: 2019ConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: Hoffmann-La RocheMore InformationMore Information: Click here to learn more. -
A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel-design Study to Assess the Efficacy, Safety, and Tolerability of AVP-786 (Deudextromethorphan Hydrobromide [d6-DM]/Quinidine Sulfate [Q]) for the Treatment of Agitation in Patients With Dementia of the Alzheimer's Type
Recruitment StatusClosedConditionCondition: Alzheimer's Disease (AD)SponsorSponsor: Avanir PharmaceuticalsMore InformationMore Information: Click here to learn more. -
New IDEAS: Imaging Dementia - Evidence for Amyloid Scanning Study: A Study to Improve Precision in Amyloid PET Coverage and Patient Care
Recruitment StatusActive: RecruitingConditionCondition: Alzheimer's Disease (AD)SponsorSponsor:More InformationMore Information: Click here to learn more. -
A Phase III, Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial to Evaluate the Safety and Efficacy of AMX0035 Versus Placebo for 48-week Treatment of Adult Patients with Amyotrophic Lateral Sclerosis (ALS)
Recruitment StatusActiveConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: Amylyx Pharmaceuticals Inc.More InformationMore Information: Click here to learn more. -
Evaluation of the Safety, Tolerability, Efficacy and Activity of AMX0035, a Fixed Combination of Phenylbutyrate (PB) and Tauroursodeoxycholic Acid (TUDCA), for the Treatment of ALS
Recruitment StatusClosedConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: Amylyx Pharmaceuticals Inc.More InformationMore Information: Click here to learn more. -
Effects of Oral Levosimendan (ODM-109) on Respiratory Function in Patients With ALS
Recruitment StatusClosedConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: Orion Corporation, Orion PharmaMore InformationMore Information: Click here to learn more. -
Analysis of Transcriptional Changes in Blood Samples from Patients with Neurologic Diseases
Recruitment StatusActive: Not RecruitingConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: Neurologix FoundationMore InformationMore Information: Click here to learn more. -
Safety Study of Oral Edaravone Administered in Subjects With ALS
Recruitment StatusActive: Enrollment ClosedConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: Mitsubishi Tanabe Pharma Development America, Inc.More InformationMore Information: Click here to learn more. -
Speech Movement Patterns of Individuals with Amyotrophic Lateral Sclerosis
Recruitment StatusClosedConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: An NIH-funded project that is being conducted at the University of Texas at Dallas in collaboration with Texas Neurology. The goal of this study is to advance the diagnosis, assessment, and treatment of speech motor impairment due to ALS using novel, computational approaches.More InformationMore Information: Click here to learn more. -
Centers for Disease Control IRB - Protocol 5923.0 – “Proposal for the State and Metropolitan Area-Based ALS Surveillance”
Recruitment StatusCompletedConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor:More InformationMore Information: Click here to learn more. -
Texas Department of State Health Services IRB – # 10-036 – “Texas Amyotrophic Lateral (ALS) Surveillance Activity”
Recruitment StatusCompletedConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor:More InformationMore Information: Click here to learn more. -
ALS Patient Care Database
Recruitment StatusCompleted: 2007ConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor:More InformationMore Information: Click here to learn more. -
A Double-Blind, Randomized, Placebo-Controlled, Multicenter Study to Assess the Safety and Efficacy and to Determine the Pharmacokinetics of Two Doses of AVP-923 (Dextromethorphan/Quinidine) in the Treatment of Pseudobulbar Affect (PBA) in Patients With Amyotrophic Lateral Sclerosis (ALS) and Multiple Sclerosis (MS)
Recruitment StatusCompleted: 2009ConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: Avanir PharmaceuticalsMore InformationMore Information: Click here to learn more. -
A Multicenter, Double-Blind, Placebo-Controlled, Study to Investigate the Safety and Efficacy of Lithium in Combination With Riluzole in Volunteers With Amyotrophic Lateral Sclerosis (ALS)
Recruitment StatusCompleted: 2009ConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: Massachusetts General HospitalMore InformationMore Information: Click here to learn more. -
TIV(Tracheostomy with invasive ventilation) for Patients with ALS in Japan and the USA: A Comparitive Study
Recruitment StatusCompleted: 2011ConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: Columbia University and the MDAMore InformationMore Information: Click here to learn more. -
Clinical Trial Ceftriaxone in Subjects With Amyotrophic Lateral Sclerosis (ALS)
Recruitment StatusCompleted: 2012ConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: Massachusetts General HospitalMore InformationMore Information: Click here to learn more. -
A Randomized, Double-Blind, Placebo-Controlled, Multi-Center Study of the Safety and Efficacy of Dexpramipexole in Subjects With Amyotrophic Lateral Sclerosis
Recruitment StatusCompleted: 2012ConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: Knopp BiosciencesMore InformationMore Information: Click here to learn more. -
Collection of Blood Samples for DNA Analysis in Motor Neuron Diseases
Recruitment StatusCompleted: 2013ConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: National Institute of Neurological Disorders and Stroke (NINDS)More InformationMore Information: Click here to learn more. -
An Open-Label, Multicenter, Extension Study to Evaluate the Long-Term Safety and Efficacy of Dexpramipexole (BIIB050) in Subjects With Amyotrophic Lateral Sclerosis
Recruitment StatusCompleted: 2013ConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: Knopp BiosciencesMore InformationMore Information: Click here to learn more. -
Analysis of Transcriptional Changes in Blood Samples from Patients with Neurologic Disease
Recruitment StatusCompleted: 2013ConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: Baylor Research InstituteMore InformationMore Information: Click here to learn more. -
Northeast ALS (NEALS) Consortium: The Upper Motor Neuron Disease (UMND) Pilot Registry
Recruitment StatusCompleted: 2013ConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor:More InformationMore Information: Click here to learn more. -
A Phase IIb, Multi-National, Double-Blind, Randomized, Placebo-Controlled Study to Evaluate the Safety, Tolerability and Efficacy of CK-2017357 in Patients With Amyotrophic Lateral Sclerosis (ALS) (BENEFIT-ALS)
Recruitment StatusCompleted: 2014ConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: CytokineticsMore InformationMore Information: Click here to learn more. -
A Study to Explore the Safety and Tolerability of Acthar in Patients With Amyotrophic Lateral Sclerosis
Recruitment StatusCompleted: 2014ConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: MallinckrodtMore InformationMore Information: Click here to learn more. -
Biogen Idec Evidera ALS Study: Qualitative Investigation of Signs and Related Functional Decline in Patients with Amyotrophic Lateral Sclerosis(ALS)
Recruitment StatusCompleted: 2015ConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: Biogen Idec, Inc.More InformationMore Information: Click here to learn more. -
ALS COSMOS (ALS Cohort Study of Multicenter Oxidative Stress)
Recruitment StatusCompleted: 2016ConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: National Institute of Health (NIH)More InformationMore Information: Click here to learn more. -
A Phase 3, Multi-National, Double-Blind, Randomized, Placebo-Controlled, Stratified, Parallel Group, Study to Evaluate the Safety, Tolerability and Efficacy of Tirasemtiv in Patients With Amyotrophic Lateral Sclerosis (ALS)
Recruitment StatusCompleted: 2017ConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: CytokineticsMore InformationMore Information: Click here to learn more. -
A Phase 3, Open-Label Extension Study of Tirasemtiv for Patients With Amyotrophic Lateral Sclerosis (ALS) Who Completed VITALITY-ALS (CY 4031)
Recruitment StatusCompleted: 2018ConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: CytokineticsMore InformationMore Information: Click here to learn more. -
A Multi-Center, Open Label Study to Assess the Safety and Tolerability of Riluzole Oral Soluble Film in Subjects With Amyotrophic Lateral Sclerosis Over 12 Weeks of Twice Daily Treatment.
Recruitment StatusCompleted: 2018ConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: Aquestive TherapeuticsMore InformationMore Information: Click here to learn more. -
Open-Label Study to Evaluate Safety, Tolerability and Pharmacokinetics of Multiple Doses of BHV-0223 in Subjects With Amyotrophic Lateral Sclerosis
Recruitment StatusCompleted: 2018ConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: Biohaven Pharmaceuticals, Inc.More InformationMore Information: Click here to learn more. -
A Multicenter, Double Blind, Placebo Controlled Study to Assess the Efficacy and Safety of H.P. Acthar® Gel in the Treatment of Subjects With Amyotrophic Lateral Sclerosis
Recruitment StatusCompleted: 2019ConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: MallinckrodtMore InformationMore Information: Click here to learn more. -
A Phase 2, Multi-Center, Double-Blind, Randomized, Dose-Ranging, Placebo-Controlled Study to Evaluate the Efficacy, Safety and Tolerability of CK-2127107 in Patients With Amyotrophic Lateral Sclerosis (ALS)
Recruitment StatusCompleted: 2019ConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: CytokineticsMore InformationMore Information: Click here to learn more. -
Answer ALS -Creation of a Large Bio-repository of iPS Cells, Cell Lines, and Bio-fluid Samples, Combined With Clinical Information to Rapidly Advance Therapeutics That Could Treat ALS
Recruitment StatusCompleted: 2020ConditionCondition: Amyotrophic Lateral Sclerosis (ALS)SponsorSponsor: Johns Hopkins UniversityMore InformationMore Information: Click here to learn more. -
A Phase 2a Open-Label, Multi-Center Study to Evaluate the Safety and Tolerability of Multiple Doses of AT-1501 in Adults with ALS
Recruitment StatusClosedConditionCondition:SponsorCondition: Amyotrophic Lateral Sclerosis (ALS)More InformationMore Information: Click here to learn more. -
A Phase 3, Randomized, Double-Blind, Placebo-Controlled, Parallel Group, Multi-Center Trial to Evaluate the Efficacy and Safety of a Single Treatment of DaxibotulinumtoxinA for Injection in Adults With Isolated Cervical Dystonia (ASPEN-1)
Recruitment StatusClosedConditionCondition: Cervical Dystonia/Spasmodic TorticollisSponsorSponsor: Revance Therapeutics, Inc.More InformationMore Information: Click here to learn more. -
A Phase 3, Open-Label, Multi-Center Trial to Evaluate the Long-Term Safety and Efficacy of Repeat Treatments of DaxibotulinumtoxinA for Injection in Adults With Isolated Cervical Dystonia (ASPEN-OLS)
Recruitment StatusClosedConditionCondition: Cervical Dystonia/Spasmodic TorticollisSponsorSponsor: Revance Therapeutics, Inc.More InformationMore Information: Click here to learn more. -
Efficacy and Safety of Eslicarbazepine Acetate (BIA 2-093) as Adjunctive Therapy for Refractory Partial Seizures in a Double-blind, Randomised, Placebo-controlled, Parallel-group, Multicentre Trial
Recruitment StatusClosedConditionCondition: Epilepsy (Epilepsy)SponsorSponsor: Bial - Portela C S.A.More InformationMore Information: Click here to learn more. -
PROSPECTIVE RANDOMIZED 12-WEEK CONTROLLED STUDY OF VISUAL FIELD CHANGE IN SUBJECTS WITH PARTIAL SEIZURES RECEIVING PREGABALIN OR PLACEBO
Recruitment StatusClosedConditionCondition: Epilepsy (Epilepsy)SponsorSponsor: PfizerMore InformationMore Information: Click here to learn more. -
Double-Blind, Randomized, Historical Control Study of the Safety and Efficacy of Eslicarbazepine Acetate Monotherapy in Subjects With Partial Epilepsy Not Well Controlled by Current Antiepileptic Drugs
Recruitment StatusCompleted: 2013ConditionCondition: Epilepsy (Epilepsy)SponsorSponsor: SunovionMore InformationMore Information: Click here to learn more. -
An Open-label, Multinational, Multicenter, Follow-up Study to Evaluate the Long-term Safety and Efficacy of Brivaracetam Used at a Flexible Dose up to a Maximum of 200 mg/Day in Subjects Aged 16 Years or Older Suffering From Epilepsy.
Recruitment StatusCompleted: 2017ConditionCondition: Epilepsy (Epilepsy)SponsorSponsor: UCB PharmaMore InformationMore Information: Click here to learn more. -
Long Term Eslicarbazepine Acetate Extension Study
Recruitment StatusCompleted: 2017ConditionCondition: Epilepsy (Epilepsy)SponsorSponsor: SunovionMore InformationMore Information: Click here to learn more. -
A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Crossover Study To Evaluate The Efficacy And Safety Of Zolmitriptan Nasal Spray For The Treatment Of Acute Migraine In Subjects Ages 6 To 11 Years, With An Open-Label Extension
Recruitment StatusClosedConditionCondition: Migraine (headache)SponsorSponsor: Impax Laboratories, LLCMore InformationMore Information: Click here to learn more. -
A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate the Efficacy, Safety, and Tolerability of Atogepant for the Prevention of Chronic Migraine (Progress)
Recruitment StatusActive: RecruitingConditionCondition: MigraineSponsorSponsor: AllerganMore InformationMore Information: Click here to learn more. -
A 12-month Prospective, Randomized, Interventional, Global, Multi-center, Activecontrolled Study Comparing Sustained Benefit of Two Treatment Paradigms (Erenumab qm vs. Oral Prophylactics) in Adult Episodic Migraine Patients
Recruitment StatusActive: RecruitingConditionCondition: MigraineSponsorSponsor: AmgenMore InformationMore Information: Click here to learn more. -
A Phase 4, Randomized, Double-blind, Placebo-controlled, Parallel-group Study to Evaluate the Efficacy and Safety of Erenumab in Adults With Chronic Migraine and Medication Overuse Headache
Recruitment StatusClosedConditionCondition: MigraineSponsorSponsor:More InformationMore Information: Click here to learn more. -
NINDS Clinical Research Collaboration Chronic Migraine Treatment Trial
Recruitment StatusCompleted: 2010ConditionCondition: MigraineSponsorSponsor: Anne LindbladMore InformationMore Information: Click here to learn more. -
An Open-Label Study to Evaluate the Safety of NP101, a Sumatriptan Iontophoretic Transdermal Patch, in the Treatment of Acute Migraine Over 12 Months
Recruitment StatusCompleted: 2010ConditionCondition: MigraineSponsorSponsor: NuPathe Inc.More InformationMore Information: Click here to learn more. -
Effect of Septal Closure of Atrial PFO on Events of Migraine With Premere: ESCAPE Migraine Trial
Recruitment StatusCompleted: 2012ConditionCondition: MigraineSponsorSponsor: Abbott Medical DevicesMore InformationMore Information: Click here to learn more. -
A Phase 2, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Efficacy and Safety of AMG 334 in Chronic Migraine Prevention
Recruitment StatusCompleted: 2016ConditionCondition: MigraineSponsorSponsor: AmgenMore InformationMore Information: Click here to learn more. -
An Open-label Extension (OLE) Study to Assess the Long-term Safety and Efficacy of AMG 334
Recruitment StatusCompleted: 2017ConditionCondition: MigraineSponsorSponsor: AmgenMore InformationMore Information: Click here to learn more. -
A Multicenter, Prospective, Randomized, Open-label Study to Compare the Efficacy, Safety, and Tolerability of BOTOX® and Topiramate for Headache Prophylaxis in Adults With Chronic Migraine
Recruitment StatusCompleted: 2017ConditionCondition: MigraineSponsorSponsor: AllerganMore InformationMore Information: Click here to learn more. -
A Phase 3, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Efficacy and Safety of AMG 334 in Migraine Prevention
Recruitment StatusCompleted: 2017ConditionCondition: MigraineSponsorSponsor: AmgenMore InformationMore Information: Click here to learn more. -
A Phase 3, Multicenter, Randomized, Double-Blind, Placebo Controlled Single Attack Study to Evaluate the Efficacy, Safety, and Tolerability of Oral Ubrogepant in the Acute Treatment of Migraine
Recruitment StatusCompleted: 2018ConditionCondition: MigraineSponsorSponsor: AllerganMore InformationMore Information: Click here to learn more. -
A Multicenter, Randomized, Open-Label Extension Study to Evaluate the Long-Term Safety and Tolerability of Oral Ubrogepant in the Acute Treatment of Migraine With or Without Aura
Recruitment StatusCompleted: 2018ConditionCondition: MigraineSponsorSponsor: AllerganMore InformationMore Information: Click here to learn more. -
A Parallel Group, Double-Blind, Randomized, Placebo Controlled Phase 3 Trial to Evaluate the Efficacy and Safety of ALD403 Administered Intravenously in Patients With Chronic Migraine
Recruitment StatusCompleted: 2018ConditionCondition: MigraineSponsorSponsor: Alder Biopharmaceuticals, Inc.More InformationMore Information: Click here to learn more. -
A Phase 2, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Efficacy and Safety of AMG 334 in Migraine Prevention
Recruitment StatusCompleted: 2019ConditionCondition: MigraineSponsorSponsor: AmgenMore InformationMore Information: Click here to learn more. -
A Phase 2a Randomized Double-blind Placebo Controlled Study to Evaluate the Efficacy and Safety of AMG 301 in Migraine Prevention
Recruitment StatusCompleted: 2019ConditionCondition: MigraineSponsorSponsor: AmgenMore InformationMore Information: Click here to learn more. -
Long-term, Prospective,Multinational, Parallel-cohort Study Monitoring Safety in Patients With MS Newly Started With Fingolimod Once Daily or Treated With Another Approved Disease-modifying Therapy
Recruitment StatusClosedConditionCondition: MSSponsorSponsor: Novartis PharmaceuticalsMore InformationMore Information: Click here to learn more. -
A Multicenter, Randomized, Double Blind, Placebo Controlled Parallel Group, Pilot Study to Assess the Efficacy and Safety of H.P. Acthar® Gel in Subjects With Relapsing-remitting Multiple Sclerosis
Recruitment StatusClosedConditionCondition: MSSponsorSponsor: MallinckrodtMore InformationMore Information: Click here to learn more. -
A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Multicenter Study to Determine the Safety and Efficacy of Natalizumab in Subjects With Relapsing-Remitting Multiple Sclerosis
Recruitment StatusCompleted: 2005ConditionCondition: MSSponsorSponsor: BiogenMore InformationMore Information: Click here to learn more. -
A Phase II Randomized, Double-Blinded, Placebo-Controlled, Multi-Center Study of Subcutaneous Daclizumab in Patients With Active, Relapsing Forms of Multiple Sclerosis
Recruitment StatusCompleted: 2006ConditionCondition: MSSponsorSponsor: PDL BioPharma, Inc.More InformationMore Information: Click here to learn more. -
A Multicenter, Open-Label, Immunogenicity and Safety Study of Avonex® (Interferon Beta-1a) 30 mcg Administered Subcutaneously to Subjects With Relapsing Multiple Sclerosis
Recruitment StatusCompleted: 2009ConditionCondition: MSSponsorSponsor: BiogenMore InformationMore Information: Click here to learn more. -
Multicenter, Double-Blind, Placebo-Controlled, Dose-Ranging Study to Determine the Safety and Efficacy of Daclizumab HYP (DAC HYP) as a Monotherapy Treatment in Subjects With Relapsing-Remitting Multiple Sclerosis
Recruitment StatusCompleted: 2011ConditionCondition: MSSponsorSponsor: BiogenMore InformationMore Information: Click here to learn more. -
A Double-Blind, Multicenter, Extension Study to Evaluate the Safety and Efficacy of DAC HYP in Subjects With Multiple Sclerosis Who Have Completed Treatment in Study 205MS201 (SELECT)
Recruitment StatusCompleted: 2012ConditionCondition: MSSponsorSponsor: BiogenMore InformationMore Information: Click here to learn more. -
A Double-Blind, Multicenter, Extension Study to Evaluate the Safety and Efficacy of DAC HYP in Subjects With Multiple Sclerosis Who Have Completed Treatment in Study 205MS201 (SELECT)
Recruitment StatusCompleted: 2012ConditionCondition: MSSponsorSponsor: BiogenMore InformationMore Information: Click here to learn more. -
Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate the Safety and Efficacy of Two Doses of Oral Dalfampridine Extended Release Tablets (5 mg and 10 mg Twice Daily) in Patients With Multiple Sclerosis
Recruitment StatusCompleted: 2012ConditionCondition: MSSponsorSponsor: Acorda TherapeuticsMore InformationMore Information: Click here to learn more. -
A Multicenter, Retrospective, Observational Study Evaluating Real-world Clinical Outcomes in Relapsing-remitting Multiple Sclerosis Patients Who Transition From Tysabri® (Natalizumab) to Tecfidera® (Dimethyl Fumarate)
Recruitment StatusCompleted: 2015ConditionCondition: MSSponsorSponsor: BiogenMore InformationMore Information: Click here to learn more. -
A Multicenter, Open-label, Extension Study to Evaluate the Long Term Safety and Efficacy of Daclizumab High Yield Process (DAC HYP) Monotherapy in Subjects With Multiple Sclerosis Who Have Completed Treatment in Study 205MS202 (SELECTION)
Recruitment StatusCompleted: 2016ConditionCondition: MSSponsorSponsor: BiogenMore InformationMore Information: Click here to learn more. -
Safety and Efficacy of ADS-5102 (Amantadine HCl) Extended Release Capsules in Patients With Multiple Sclerosis and Walking Impairment
Recruitment StatusCompleted: 2016ConditionCondition: MSSponsorSponsor: Adamas Pharmaceuticals, Inc.More InformationMore Information: Click here to learn more. -
A Multicenter, Double- Blind, Placebo- Controlled Study of Montelukast on Gastrointestinal Tolerability in Patients With Relapsing Forms of Multiple Sclerosis Receiving Tecfidera® (Dimethyl Fumarate) Delayed Release Capsules
Recruitment StatusCompleted: 2017ConditionCondition: MSSponsorSponsor: BiogenMore InformationMore Information: Click here to learn more. -
A 12-month, Randomized, Rater- and Dose-blinded Study to Compare the Efficacy and Safety of Fingolimod 0.25 mg and 0.5 mg Administered Orally Once Daily With Glatiramer Acetate 20 mg Administered Subcutaneously Once Daily in Patients With Relapsing-remitting Multiple Sclerosis
Recruitment StatusCompleted: 2018ConditionCondition: MSSponsorSponsor: Novartis PharmaceuticalsMore InformationMore Information: Click here to learn more. -
A Phase 3, Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Safety and Efficacy of Ravulizumab in Complement-Inhibitor-Naïve Adult Patients With Generalized Myasthenia Gravis
Recruitment StatusActive: Enrollment ClosedConditionCondition: Myasthenia GravisSponsorSponsor: Alexion PharmaceuticalsMore InformationMore Information: Click here to learn more. -
Patient Assisted Intervention for Neuropathy: Comparison of Treatment in Real Life Situations (PAIN-CONTRoLS)
Recruitment StatusCompleted: 2017ConditionCondition: NeuropathySponsorSponsor: University of Kansas Medical CenterMore InformationMore Information: Click here to learn more. -
Effect of LY3154207 on Cognition in Mild-to-Moderate Dementia Due to Lewy Body Dementia (LBD) Associated With Idiopathic Parkinson's Disease (PD) or Dementia With Lewy Bodies (DLB)
Recruitment StatusClosedConditionCondition: Parkinson's Disease (PD)SponsorSponsor: Eli Lilly and CompanyMore InformationMore Information: Click here to learn more. -
This is a Multi-center, Double-blind, Three Arm, Parallel Group, Placebo-controlled, Randomized Study Designed to Evaluate the Efficacy, Safety and Tolerability of Dalfampridine.
Recruitment StatusCompleted: 2016ConditionCondition: StrokeSponsorSponsor: Acorda TherapeuticsMore InformationMore Information: Click here to learn more. -
An Extension Study to Evaluate the Long-Term Safety, Tolerability and Efficacy of Dalfampridine Extended-Release Tablets. for the Treatment of Chronic Post-Ischemic Stroke Walking Deficits .
Recruitment StatusCompleted: 2017ConditionCondition: StrokeSponsorSponsor: Acorda TherapeuticsMore InformationMore Information: Click here to learn more. -
A Phase 3, Randomized, Double-Blind Study to Evaluate the Safety and Efficacy of Elvitegravir/Emtricitabine/Tenofovir Disoproxil Fumarate/GS-9350 Versus Ritonavir-Boosted Atazanavir Plus Emtricitabine/Tenofovir Disoproxil Fumarate in HIV-1 Infected, Antiretroviral Treatment-Naive Adults
Recruitment StatusCompleted: 2014ConditionCondition: Other (HIV)SponsorSponsor: Gilead SciencesMore InformationMore Information: Click here to learn more.
Documents & Forms
- Medical Appointment Cancellation & No Show Policy
- Texas Neurology Cares Flyer
- Texas Neurology Cares Food Drive
- Texas Neurology Cares Toy Drive
- Texas Neurology Cares Winter Clothing Drive
- 52 Proven Stress Reducers
- Authorization to Disclose Protected Health Information Form (To Obtain) (Dallas Version)
- Authorization to Disclose Protected Health Information Form (To Release) (Dallas Version)
- Botox Savings Plan Overview Brochure
- Botox SPP Patient Instructions
- Buy and Bill and SPP Considerations
- Chronic Pain or Illness: Relationships and Communication
- Commonly Reported Symptoms at Various Phases of Migraine and Chronic Daily Headache
- The Complete Headache Chart
- Distinguishing Sinus Headache From Migraine
- Emergency Room Treatment Form
- Financial Policy
- Food Additives And Migraines
- General Guidelines for Treatment with Preventative Medications
- Guide to Acquiring Botox Through Specialty Pharmacy
- Headache Calendar
- Infusion Order Form (IVIG) Coming Soon
- Infusion Order Form (Lemtrada (Alamtuzumab)) Coming Soon
- Infusion Order Form (Migraine) Coming Soon
- Infusion Order Form (Ocrevus (Ocrelizumab))
- Infusion Order Form (Remicade (Infliximab)) Coming Soon
- Infusion Order Form (Tysabri (Natalizumab)) Coming Soon
- Low Tyramine Headache Diet
- Menstrually Related Migraine
- MRI Screening Form
- New Patient Packet (Dallas Version)
- Notice of Privacy Practices (English Version) (Spanish Version)
- Nurse Practitioner/Physician Assistant Information Guide
- Packet (Sleep Study)
- Patient Authorization Form for Radicava IV Infusion
- Questionnaire (Headache I)
- Questionnaire (Headache II)
- Questionnaire (Intake)
- Questionnaire (MIDAS)
- Referral Form (Diagnostic Imaging Center)
- Referral Form (General)
- Referral Form (Sleep Disorders Center)
- The Role of Triggering Factors in Migraine Headaches
- Sinus Headache or Migraine?
- Steps to Take to Reduce the Impact of Migraine at Work
- TeleVisit Terms & Conditions
- TeleVisit User Guide
- Ten Day Prednisone Program
- Tips for Better Sleep
- Tips for Improving the Inner "Landscape" or Increasing Self-Esteem
- Travel Tips for the Headache Sufferer
- Treatment Strategies and Options for Chronic Daily Headache Sufferers
- What Triggers A Migraine
- What You Need to Know about Your MRI
- Oops! It doesn't look like there's anything here right now...
- Headache Relief for Women by Dr. Alan Rappaport
- Heal Your Headache by Dr. David Buchholtz
- The Migraine Brain by Dr. Carolyn Bernstein
- Tell Me What to Eat/Headache by Elaine McGee R.D.
Resources
Information from your physicians, other MS patients and families, as well as the National MS Society are likely the most useful resources.
- Alzheimers Foundation
- American Academy of Neurology
- American Academy of Sleep Medicine
- American Council for Headache Education (ACHE)
- American Headache and Migraine Association (AHMA)
- American Medical Association
- American Parkinson Disease Association
- American Stroke Association
- Beeman Hotel Reservation Link
- BOTOX Chronic Migraine
- CenterWatch
- Clinical Trials Sites
- The Consortium of Multiple Sclerosis Centers (CMSC)
- Dystonia Medical Research Foundation
- Eunice Kennedy Shriver National Institute of Child Health and Human Development
- Everyday Life with ALS book
- The Foundation for Peripheral Neuropathy
- The Impact of Migraine: Q&A with Dr. David Dodick
- International Organization of Multiple Sclerosis Nurses (IOMSN)
- Medicare
- The Movement Disorder Society
- The Multiple Sclerosis Association of America (MSAA)
- Multiple Sclerosis International Foundation
- Muscular Dystrophy Association (MDA) - ALS Division
- MyChronicMigraine
- National ALS Registry
- National Center for Complementary and Alternative Medicine
- National Institute for Neurological Disorders and Stroke (NINDS)
- National Institute of Neurological Disorders and Stroke (NINDS) (ALS Information Page)
- Northeast ALS Consortium (NEALS)
- Parkinson Disease Foundation
- Quack Watch
- Washington University
- We Move
- Oops! It doesn't look like there's anything here right now...
